Carnot's theorem (thermodynamics)
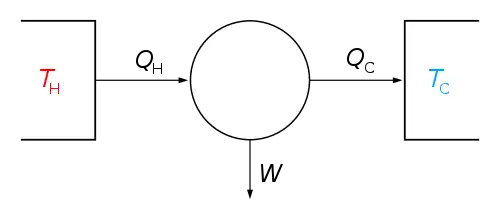
Carnot's theorem, developed in 1824 by Nicolas Léonard Sadi Carnot, also called Carnot's rule, is a principle that specifies limits on the maximum efficiency any heat engine can obtain. The efficiency of a Carnot engine depends solely on the temperatures of the hot and cold reservoirs.
| Thermodynamics |
|---|
 |
|
Carnot's theorem states that all heat engines operating between two heat reservoirs are less efficient than a Carnot heat engine operating between the same reservoirs. Every Carnot heat engine operating between a pair of heat reservoirs is equally efficient, regardless of the working substance employed or the operation details.
The maximum efficiency is the ratio of the temperature difference between the reservoirs and the temperature of the hot reservoir, expressed in the equation , where TC and TH are the absolute temperatures of the cold and hot reservoirs, respectively, and the efficiency is the ratio of the work done by the engine to the heat drawn out of the hot reservoir.
Carnot's theorem is a consequence of the second law of thermodynamics. Historically, it was based on contemporary caloric theory, and preceded the establishment of the second law.[1]
Proof

The proof of the Carnot theorem is a proof by contradiction, or reductio ad absurdum (a method to prove a statement by assuming its falsity and logically deriving a false or contradictory statement from this assumption), as illustrated by the right figure showing two heat engines operating between two thermal reservoirs at different temperature. A heat engine with greater efficiency is driving a heat engine with less efficiency , causing the latter to act as a heat pump. However, if , then the net heat flow would be backwards, i.e., into the hot thermal reservoir:
where represents heat, for input, for output, and for the hot or high temperature thermal reservoir. This means that heat into the hot reservoir from the engine pair is greater than heat into the engine pair from the hot reservoir (i.e., the hot reservoir continuously gets energy), and it is generally agreed that such a heat transfer is impossible by the second law of thermodynamics.
We begin by verifying the values of work and heat depicted in the right figure. First, we must point out an important caveat: the engine with less efficiency is driven as a heat pump by the engine with more efficiency , and therefore must be a reversible engine. If the engine is not reversible, then the device could be built, but the expressions for work and heat flow shown in the figure would not be valid.
For each engine, the absolute value of the energy entering the engine, , must equal the absolute value of the energy leaving from the engine, (Otherwise, energy is continuously accumulated in each engine or the conservation of energy is violated.):
These expressions are consistent with the definition of efficiency as for the both engines (The second equation above is derived by this efficiency definition, , and the conservation of energy on the engine .):
In these expressions, the sign convention is used (+ sign for heat entering an engine, + sign for work done by an engine to its surroundings). It may seem odd that a hypothetical heat pump with low efficiency is being used to violate the second law of thermodynamics, but the figure of merit for refrigerator units is not efficiency, , but the coefficient of performance (COP),[2] which is . A reversible heat engine with low thermodynamic efficiency delivers more heat to the hot reservoir for a given amount of work when it is being driven as a heat pump.
Having established that the heat values shown in the right figure are correct, Carnot's theorem may be proven for irreversible and the reversible heat engines.[3]
Reversible engines
To see that every reversible engine operating between reservoirs at temperatures and must have the same efficiency, assume that two reversible heat engines have different values of efficiency , and let the more efficient engine drive the less efficient engine as a heat pump. As the figure shows, this will cause heat to flow from the cold to the hot reservoir without any external work or energy (from other than the reservoirs and the engines), which violates the second law of thermodynamics. Therefore, both (reversible) heat engines have the same efficiency, and we conclude that:
- All reversible engines that operate between the same two thermal (heat) reservoirs have the same efficiency.
This is an important result because it helps establish the Clausius theorem, which implies that the change in entropy is unique for all reversible processes:[4]
as the entropy change is the same over all reversible process paths from a state to a state in a V-T (Volume-Temperature) space. If this integral were not path independent, then entropy would not be a state variable.[5]
Irreversible engines
If one of the engines is irreversible, then it must be the engine , placed so that it reversely drives the less efficient but reversible engine . But if this irreversible engine is more efficient than the reversible engine, (i.e., if ), then the second law of thermodynamics is violated. Since a Carnot heat engine operating in a Carnot cycle is a reversible engine, we have the first part of Carnot's theorem:
- No irreversible engine is more efficient than a Carnot engine operating between the same two thermal reservoirs.
Definition of thermodynamic temperature
The efficiency of the engine is the work divided by the heat introduced to the system or
-
(1)
where wcy is the work done per cycle. Thus, the efficiency depends only on qC / qH.[6]
Because all reversible engines operating between the same heat reservoirs are equally efficient, all reversible heat engines operating between temperatures T1 and T2 must have the same efficiency, meaning the efficiency is a function only of the two temperatures:
-
(2)
In addition, a reversible heat engine operating between temperatures T1 and T3 must have the same efficiency as one consisting of two cycles, one between T1 and another (intermediate) temperature T2, and the second between T2 and T3. This can only be the case if
Specializing to the case that is a fixed reference temperature: the temperature of the triple point of water. Then for any T2 and T3,
Therefore, if thermodynamic temperature is defined by
then the function viewed as a function of thermodynamic temperature, is
and the reference temperature T1 has the value 273.16. (Of course any reference temperature and any positive numerical value could be used—the choice here corresponds to the Kelvin scale.)
It follows immediately that
-
(3)
Substituting Equation 3 back into Equation 1 gives a relationship for the efficiency in terms of temperature:
-
(4)
Applicability to fuel cells and batteries
Since fuel cells and batteries can generate useful power when all components of the system are at the same temperature (), they are clearly not limited by Carnot's theorem, which states that no power can be generated when . This is because Carnot's theorem applies to engines converting thermal energy to work, whereas fuel cells and batteries instead convert chemical energy to work.[7] Nevertheless, the second law of thermodynamics still provides restrictions on fuel cell and battery energy conversion.[8]
A Carnot battery is a type of energy storage system that stores electricity in heat storage and converts the stored heat back to electricity through thermodynamic cycles.[9]
References
- John Murrell (2009). "A Very Brief History of Thermodynamics". Retrieved May 2, 2014. Archive copy at the Internet Archive PDF (142 Archived November 22, 2009, at the Wayback Machine KB)
- Tipler, Paul; Mosca, G. (2008). "19.2, 19.7". Physics for Scientists and Engineers (6th ed.). Freeman. ISBN 9781429201322.
- "Lecture 10: Carnot theorem" (PDF). Feb 7, 2005. Retrieved October 5, 2010.
- Ohanian, Hans (1994). Principles of Physics. W.W. Norton and Co. p. 438. ISBN 039395773X.
- http://faculty.wwu.edu/vawter/PhysicsNet/Topics/ThermLaw2/ThermalProcesses.html Archived 2013-12-28 at the Wayback Machine, and http://www.itp.phys.ethz.ch/education/hs10/stat/slides/Laws_TD.pdf Archived 2013-12-13 at the Wayback Machine. Both retrieved 13 December 2013.
- The sign of qC > 0 for the waste heat lost by the system violates the sign convention of heat.
- "Fuel Cell versus Carnot Efficiency". Retrieved Feb 20, 2011.
- Jacob, Kallarackel T; Jain, Saurabh (July 2005). Fuel cell efficiency redefined : Carnot limit reassessed. Q1 - Ninth International Symposium on Solid Oxide Fuel Cells (SOFC IX). USA. Archived from the original on 2016-03-04. Retrieved 2013-04-23.
- Dumont, Olivier; Frate, Guido Francesco; Pillai, Aditya; Lecompte, Steven; De paepe, Michel; Lemort, Vincent (2020). "Carnot battery technology: A state-of-the-art review". Journal of Energy Storage. 32: 101756. doi:10.1016/j.est.2020.101756. ISSN 2352-152X.